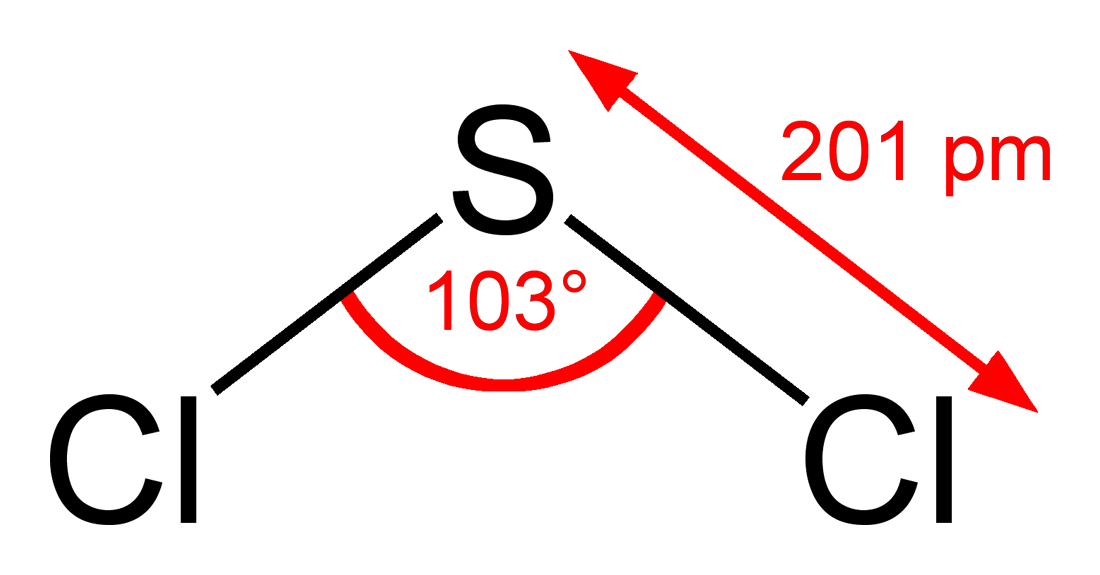
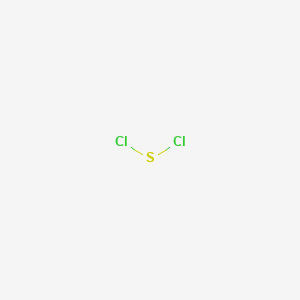
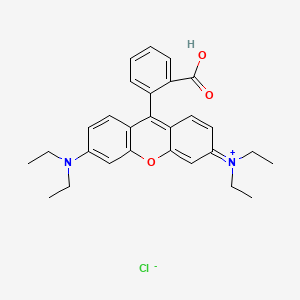
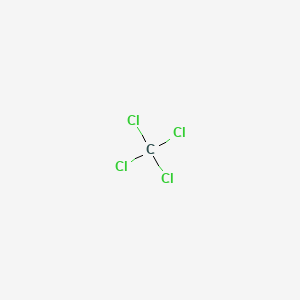
Sulfur Dichloride At Room Temperature
The scl 2 must be applied at room temperature as quickly as possible after distillation within one hour.
Sulfur dichloride at room temperature. Separation of scl 2 from s 2 cl 2 is possible via distillation with pcl 3 to form an azeotrope of 99 purity however sulfur dichloride loses chlorine slowly at room temperature and reverts to disulfur dichloride. Disulfur dichloride s 2 cl 2 is the most common impurity in scl 2. Sulfur dichloride solvent in hexane carbon. It is produced by partial chlorination of elemental sulfur.
It is used in organic synthesis and adds chloride substituted thioethers to alkenes. The reaction proceeds at usable rates at room temperature. With water the dichloride structures sulfuric corrosive sulfur and a blend of thionic acids h 2 s x o y. Sulfur dichloride can be purified by distillation in the presence of stabilizers.
Diiminosuccinonitrile reacts with sulfur dichloride in dichloromethane at room temperature to give 1 2 5 thiadiazole 3 4 dicarbonitrile 226 in 93 yield 1972joc4136 the addition of catalytic naked chloride to the reaction mixture gave the bi 1 2 5 thiadiazole 227 1991cb1517 no experimental data were given for this transformation but it was shown that the bi 1 2 5 thiadiazole 227 can. S 8 4 cl 2 4 s 2 cl 2 δh 58 2 kj mol. In the laboratory chlorine gas is led into a flask containing elemental sulfur. Technical grade sulfur dichloride is produced by passing gaseous chlorine into s2cl2 at low temperature until the correct chlorine content is reached.
Disulfur dichloride s 2 cl 2 is the most common impurity in scl 2.